Ríos, M.-C.; Portilla, J. Recent Advances in Synthesis and Properties of Pyrazoles. Chemistry (Easton). 2022, 4 (3), 940–968. https://doi.org/10.3390/chemistry4030065.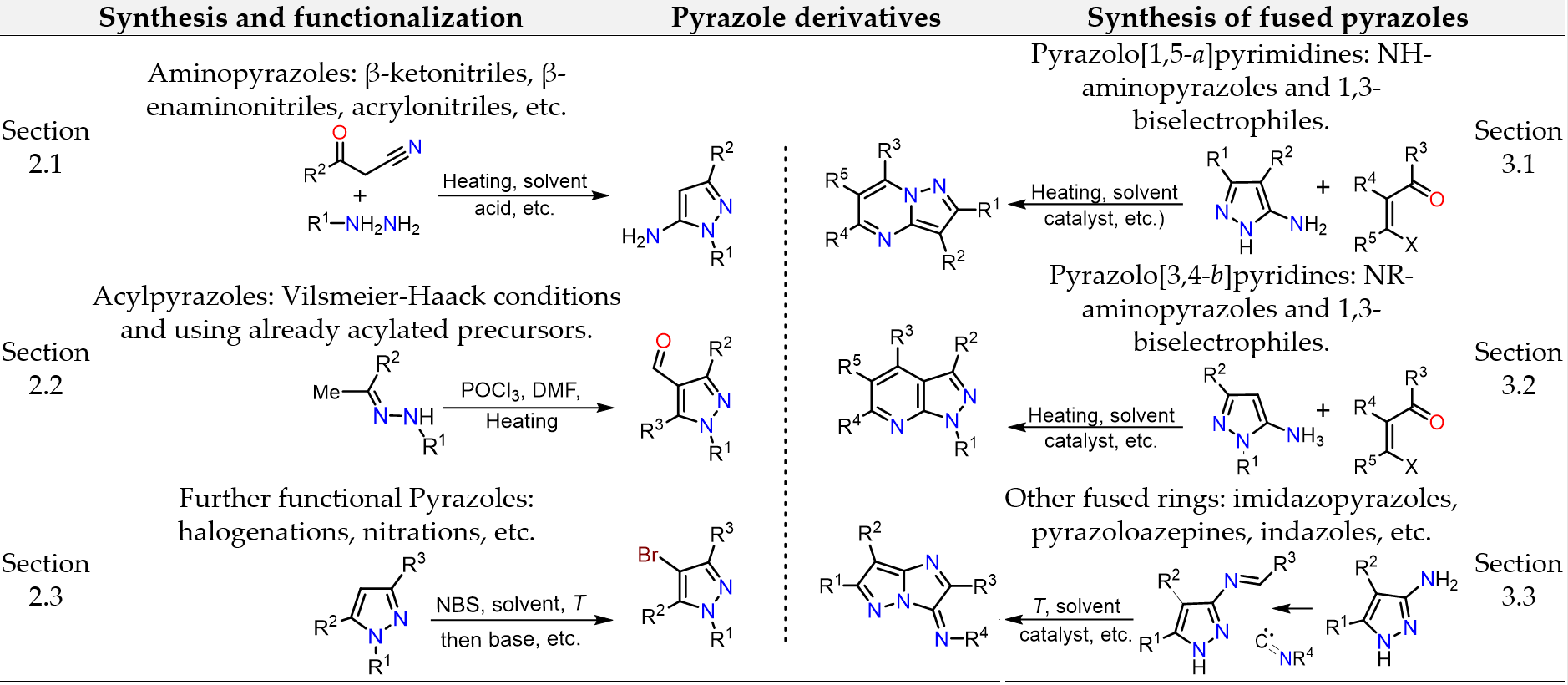
Ballesteros-Casallas, A.; Paulino, M.; Vidossich, P.; Melo, C.; Jiménez, E.; Castillo, J.-C.; Portilla, J.; Miscione, G. Pietro. Synthesis of 2,7-Diarylpyrazolo[1,5-a]Pyrimidine Derivatives with Antitumor Activity. Theoretical Identification of Targets. Eur. J. Med. Chem. Reports 2022, 4 (2022), 100028. https://doi.org/10.1016/j.ejmcr.2021.100028. 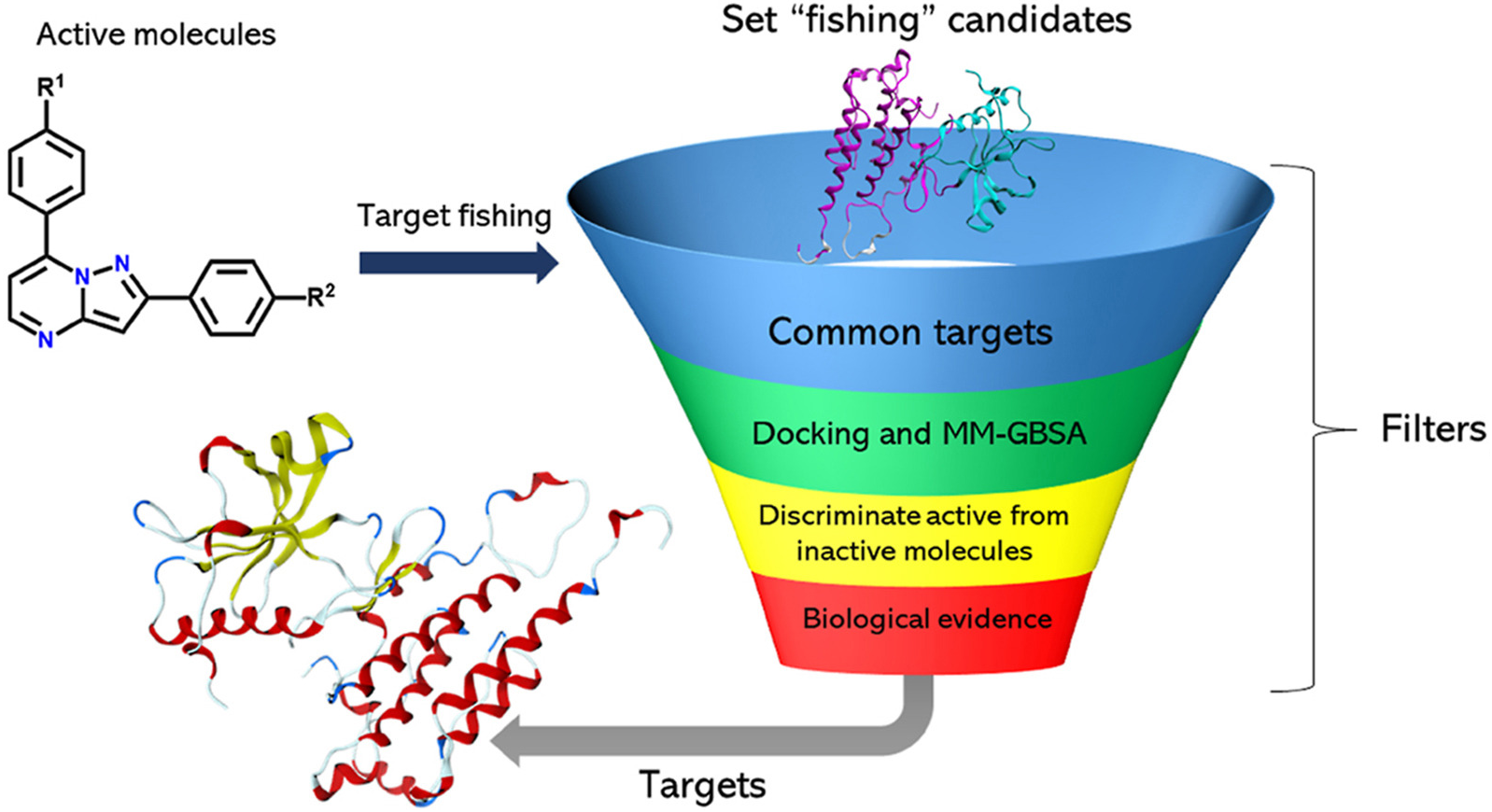
Tigreros, A.; Macías, M.; Portilla, J. Expeditious Ethanol Quantification Present in Hydrocarbons and Distilled Spirits: Extending Photophysical Usages of the Pyrazolo[1,5-a]Pyrimidines. Dye. Pigment. 2022, 202, 110299. https://doi.org/https://doi.org/10.1016/j.dyepig.2022.110299. 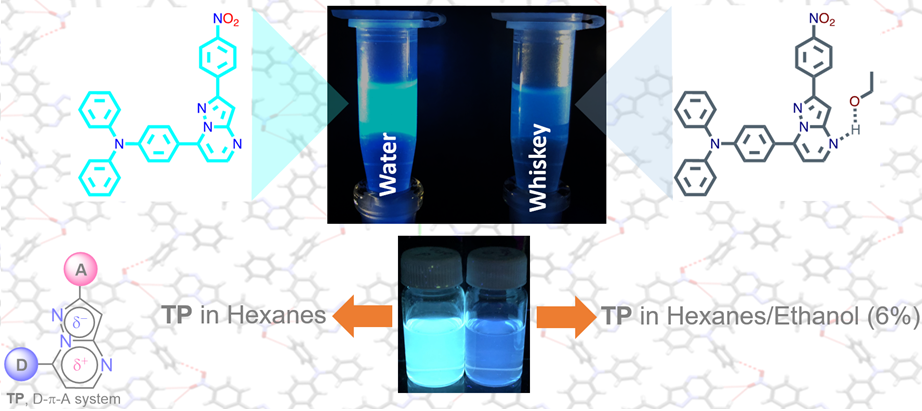
Tigreros, A.; Portilla, J. Ecological and Economic Efforts in the Development of Molecular Sensors for the Optical Detection of Cyanide Ions. Eur. J. Org. Chem. 2022, 2022 (19), e202200249. https://doi.org/10.1002/ejoc.202200249. 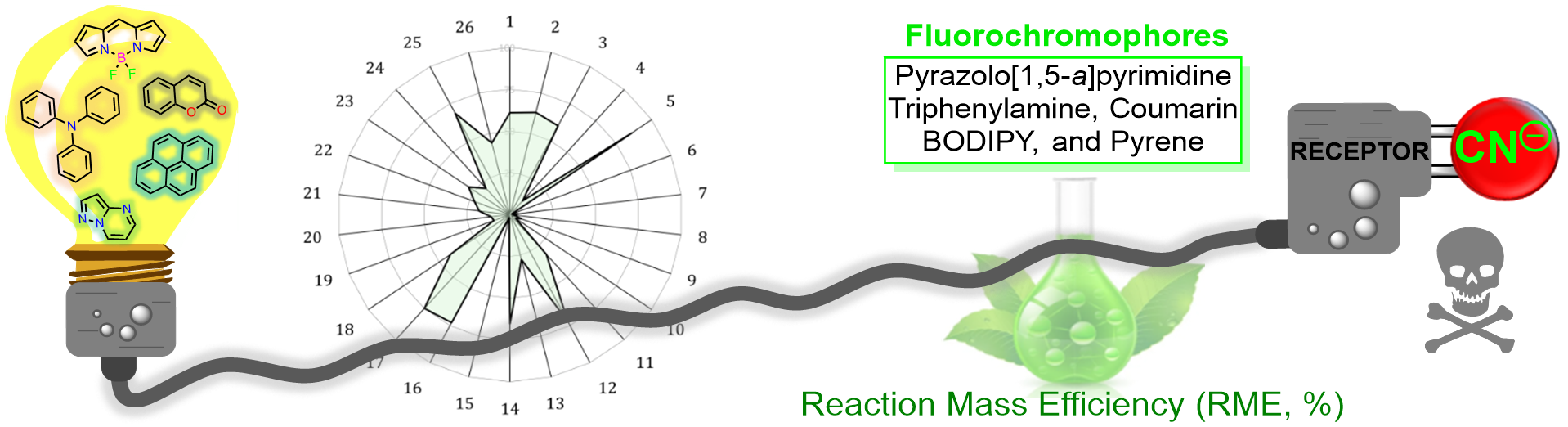
Becerra, D.; Portilla, J.; Cobo, J.; Castillo, J. C.; Macías, M. A. The Effect of Molecular Planarity and Resonant Effects on Supramolecular Structures of N-(5-Pyrazolyl)Imines by X-Ray Crystallographic Analysis. J. Mol. Struct. 2022, 1252, 132098. https://doi.org/10.1016/j.molstruc.2021.132098. 
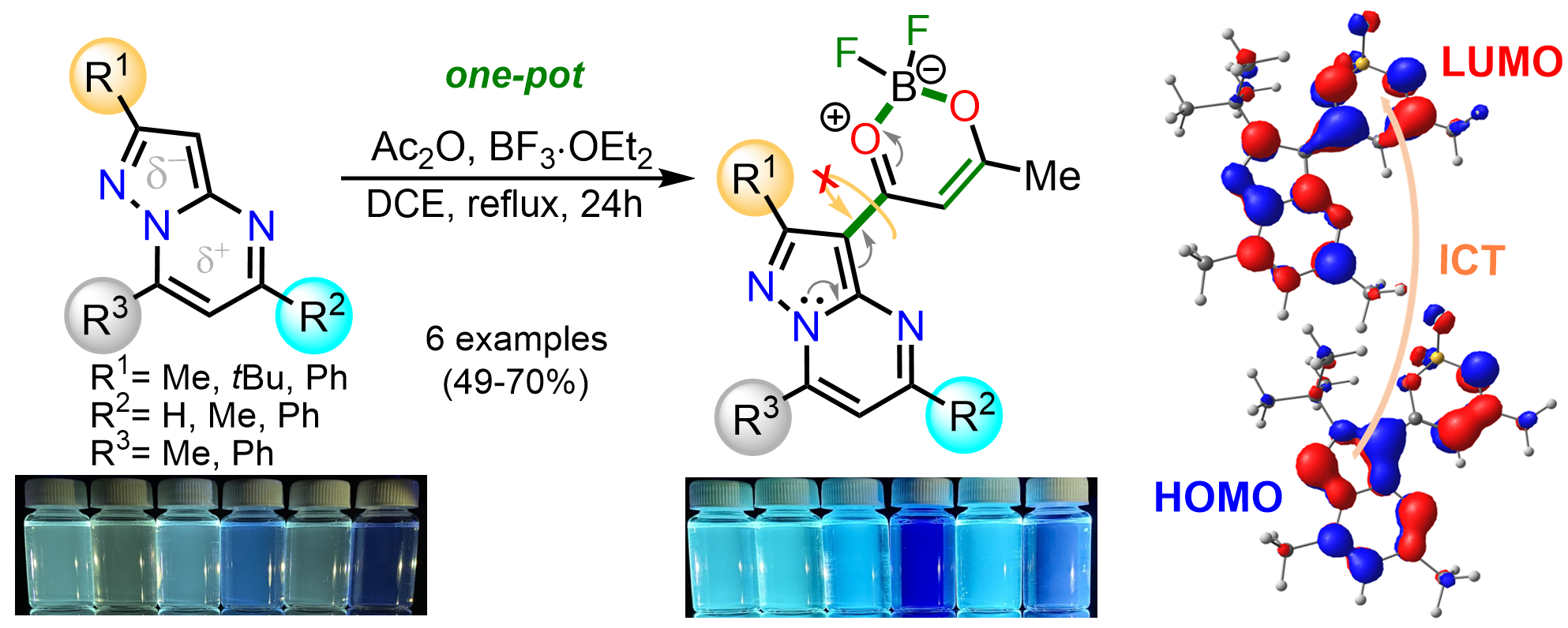
Aranzazu, S.; Tigreros, A.; Arias-G, A.; Zapata-rivera, J.; Portilla, J. BF3‑Mediated Acetylation of Pyrazolo[1,5‑a]Pyrimidines and Other Π‑Excedent (N‑Hetero)Arenes. J. Org. Chem. 2022, 87 (15), 9839–9850. https://doi.org/10.1021/acs.joc.2c00881. 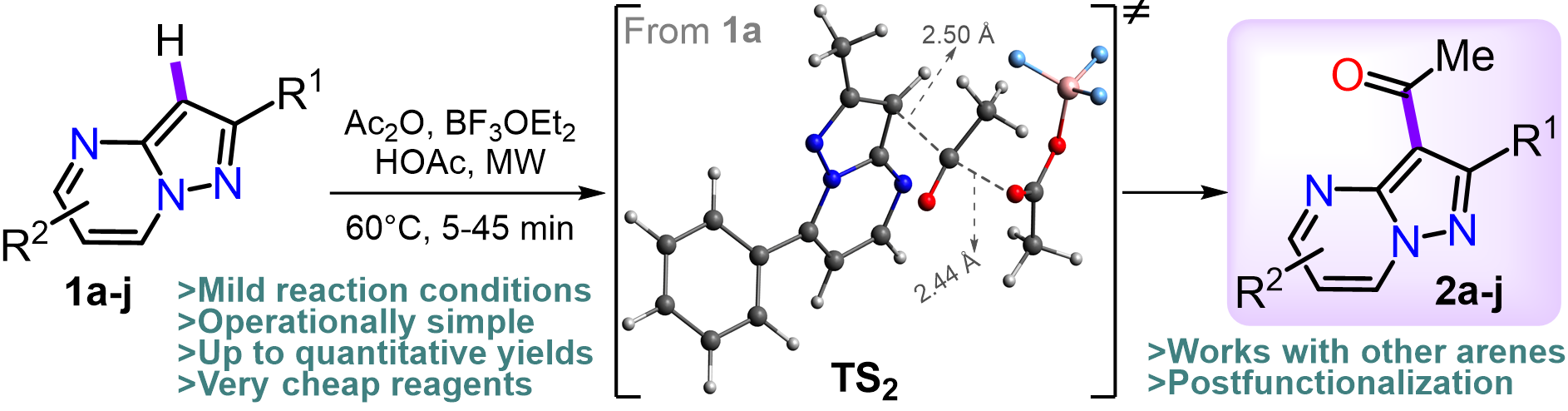
Phenylsulfonyl, H.; Salinas-torres, A.; Portilla, J.; Rojas, H.; Becerra, D.; Castillo, J. Synthesis, Spectroscopic Analysis, and In Vitro Anticancer. Molbank 2022, 2022, M1387. https://doi.org/10.3390/M1387. 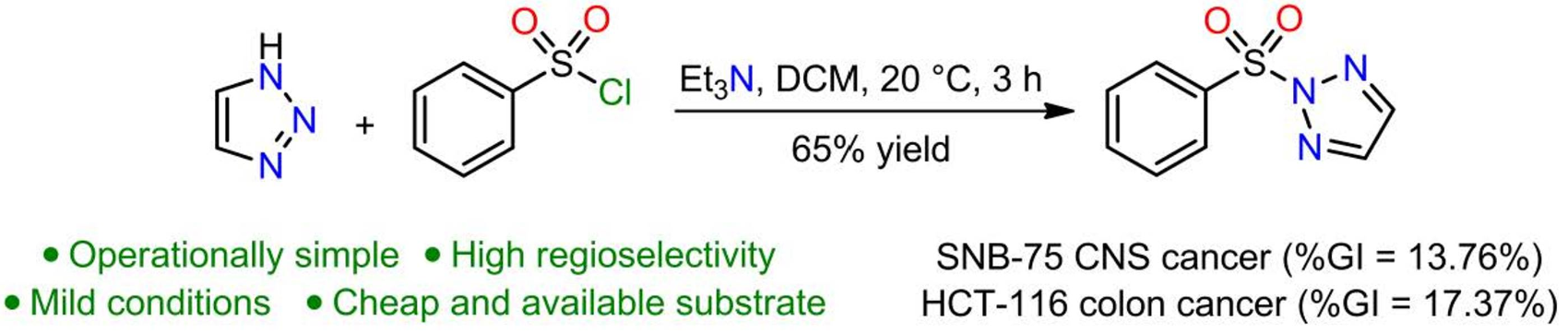
Elejalde-Cadena, N. R.; García-Olave, M.; Figueroa, D.; Vidossich, P.; Miscione, G. Pietro; Portilla, J. Influence of Steric Effect on the Pseudo-Multicomponent Synthesis of N-Aroylmethyl-4-Arylimidazoles. Molecules 2022, 27 (4), 1165. https://doi.org/10.3390/molecules27041165. 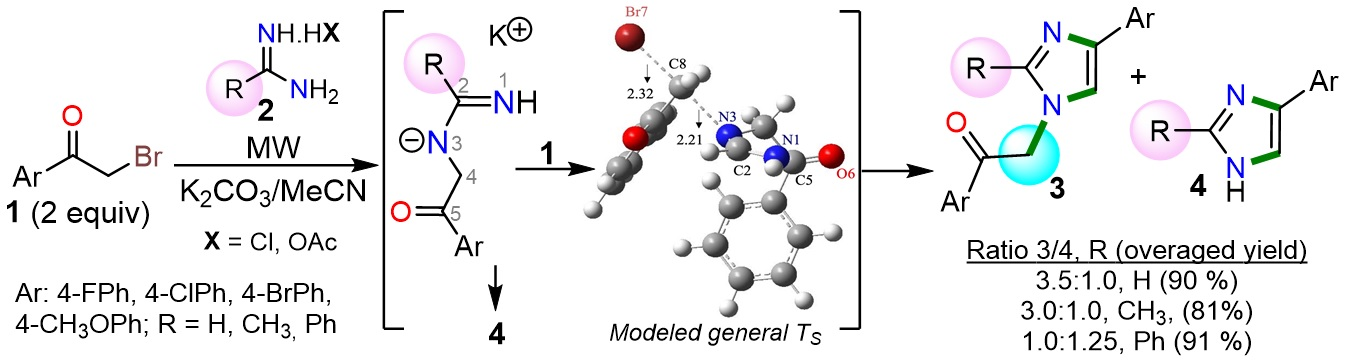
Mancipe, S.; Castillo, J. C.; Brijaldo, M. H.; López, V. P.; Rojas, H.; Macías, M. A.; Portilla, J.; Romanelli, G. P.; Martínez, J. J.; Luque, R. B-Containing Hydrotalcites Effectively Catalyzed Synthesis of 3-(Furan-2-Yl)Acrylonitrile Derivatives via the Knoevenagel Condensation. ACS Sustain. Chem. Eng. 2022, 10 (38), 12602–12612. https://doi.org/10.1021/acssuschemeng.2c03209. 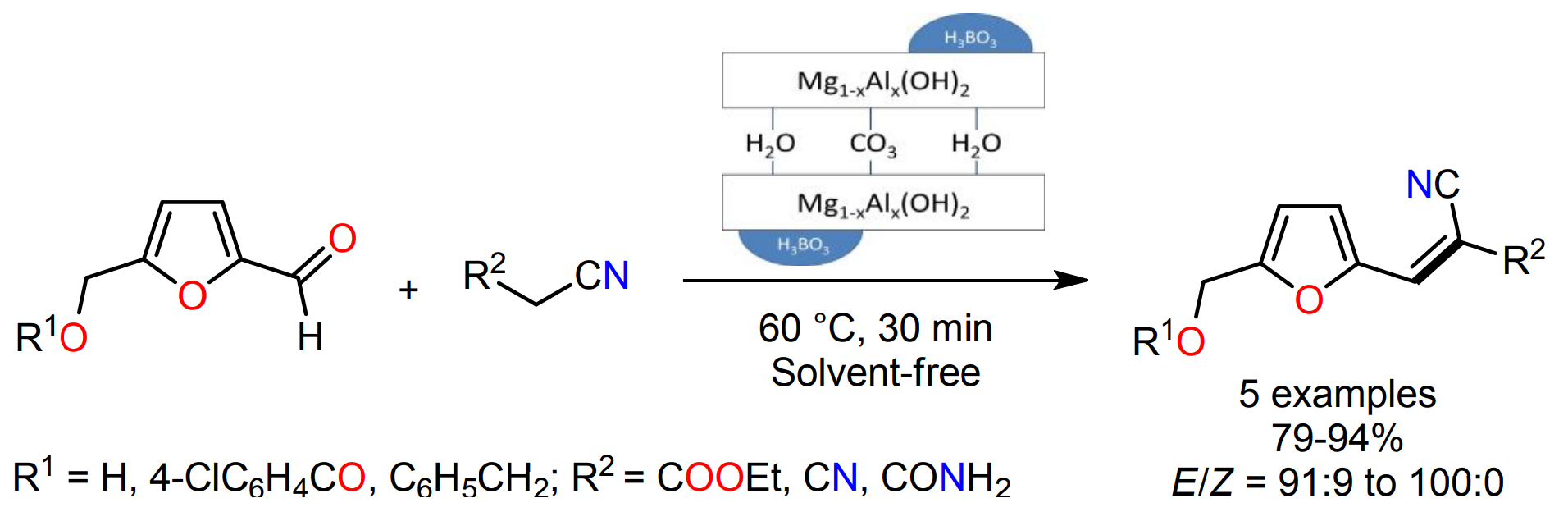
Tigreros, A.; Macías, M.; Portilla, J. Structural, Photophysical, and Water Sensing Properties of Pyrazolo[1,5‐a]Pyrimidine‐Triphenylamine Hybrid Systems. ChemPhotoChem 2022, 2022, e202200133. https://doi.org/10.1002/cptc.202200133. 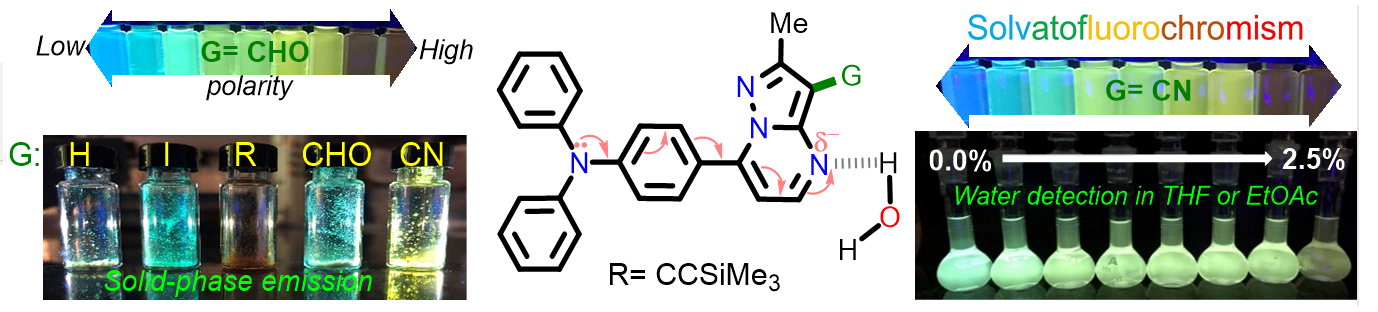
Sarmiento, J. T.; Portilla, J. Current Advances in Chemosensors Diazoles-Based for CN– and F– Detection. Curr. Org. Synth. 2023, 20 (1), 61–79. https://doi.org/10.2174/1570179419666220218095741.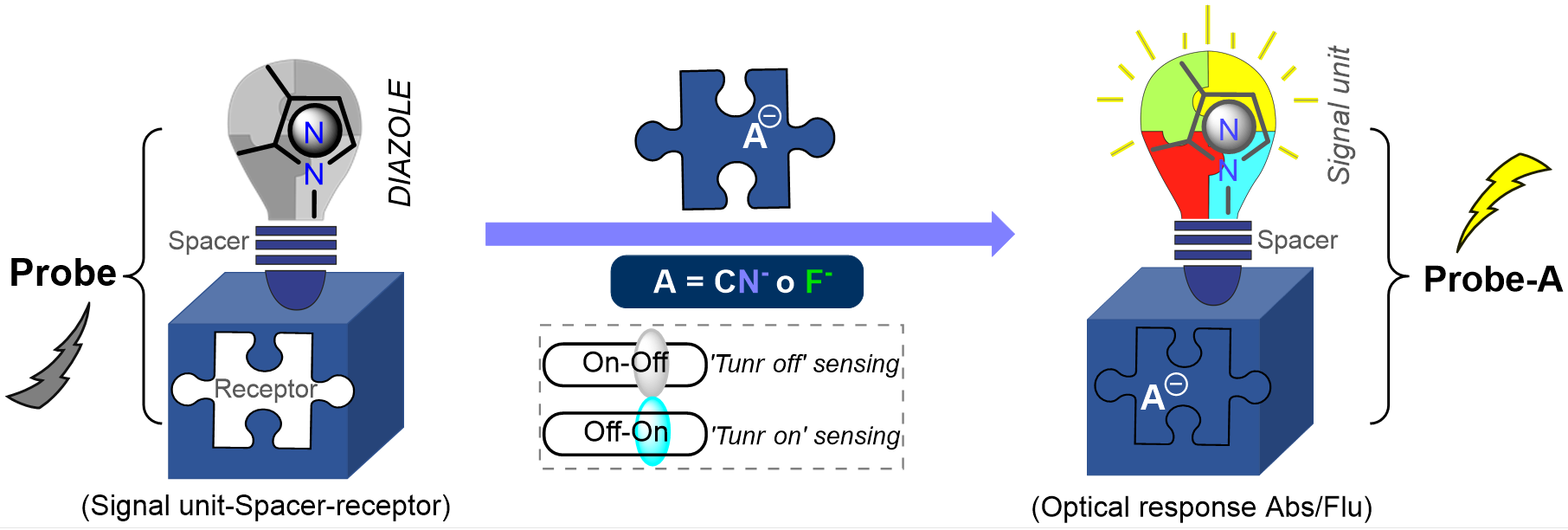
García-Olave, M.; Elejalde-cadena, N. R.; Portilla, J.; Macías, M. A. C – H ··· X ( X = F , Cl ) and Cl ··· Cl Halogen-Me Diate d Interactions Driving the Crystal Packing in N -Substituted 4-Arylimidazoles. J. Mol. Struct. 2023, 1272, 134181. https://doi.org/10.1016/j.molstruc.2022.134181.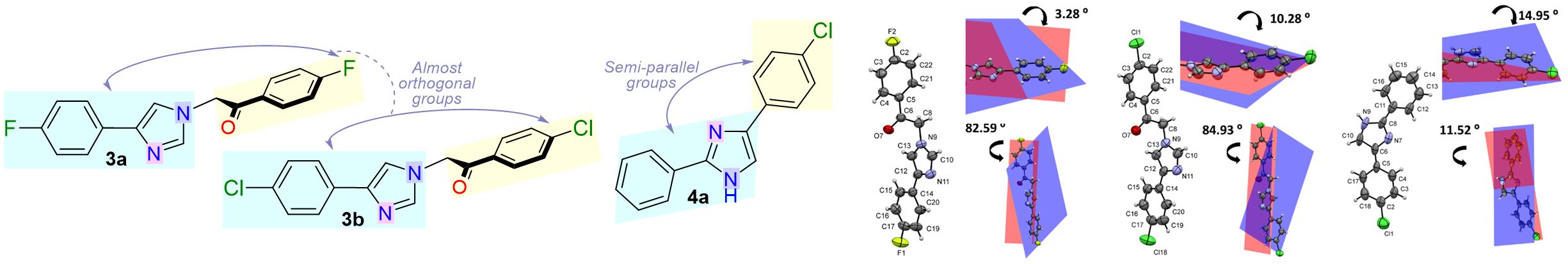
Tigreros, A.; Bedoya-Malagón, C.; Valencia, A.; Núñez-Portela, M.; Portilla, J. Photophysical and anion sensing properties of a triphenylamine–dioxaborinine trimeric compound. RSC Adv. 2023, 13, 1757-1764. https://doi.org/10.1039/D2RA07498B.
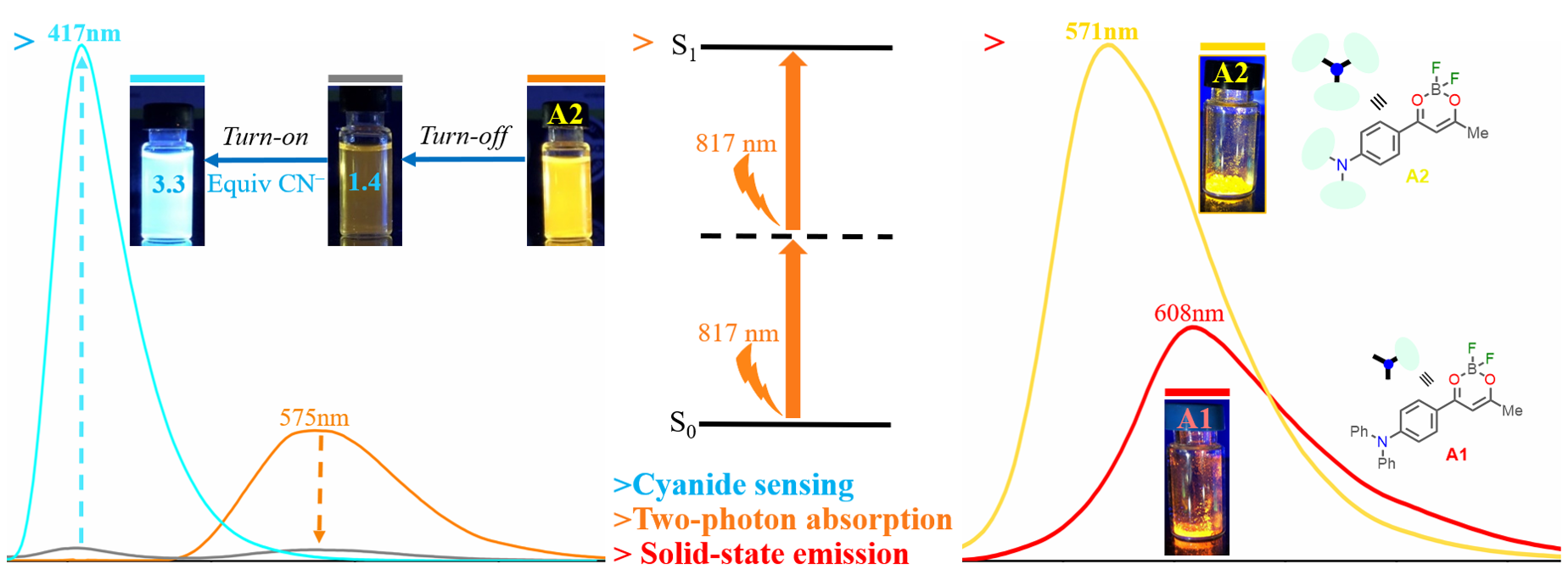
Tigreros, A.; Aranzazu, S.; Ríos, M.-C.; Portilla, J. Pyrazolo[1,5-a]pyrimidine-Dioxaborinine Hybrid Dyes: Synthesis and Substituent Effect in the Photophysical Properties. Eur. J. Org. Chem. 2023, 26 (15), e202300089. https://doi.org/10.1002/ejoc.202300089.
Elejalde-cadena, N. R.; García-Olave, M.; Macías, M. A.; Portilla, J. Influence of halogen atoms and hydrogen bonds in the crystal structure of 1,2,4-trisubstituted imidazoles having haloaryl groups. J. Mol. Struct. 2023, 1286, 135662. https://doi.org/10.1016/j.molstruc.2023.135662.
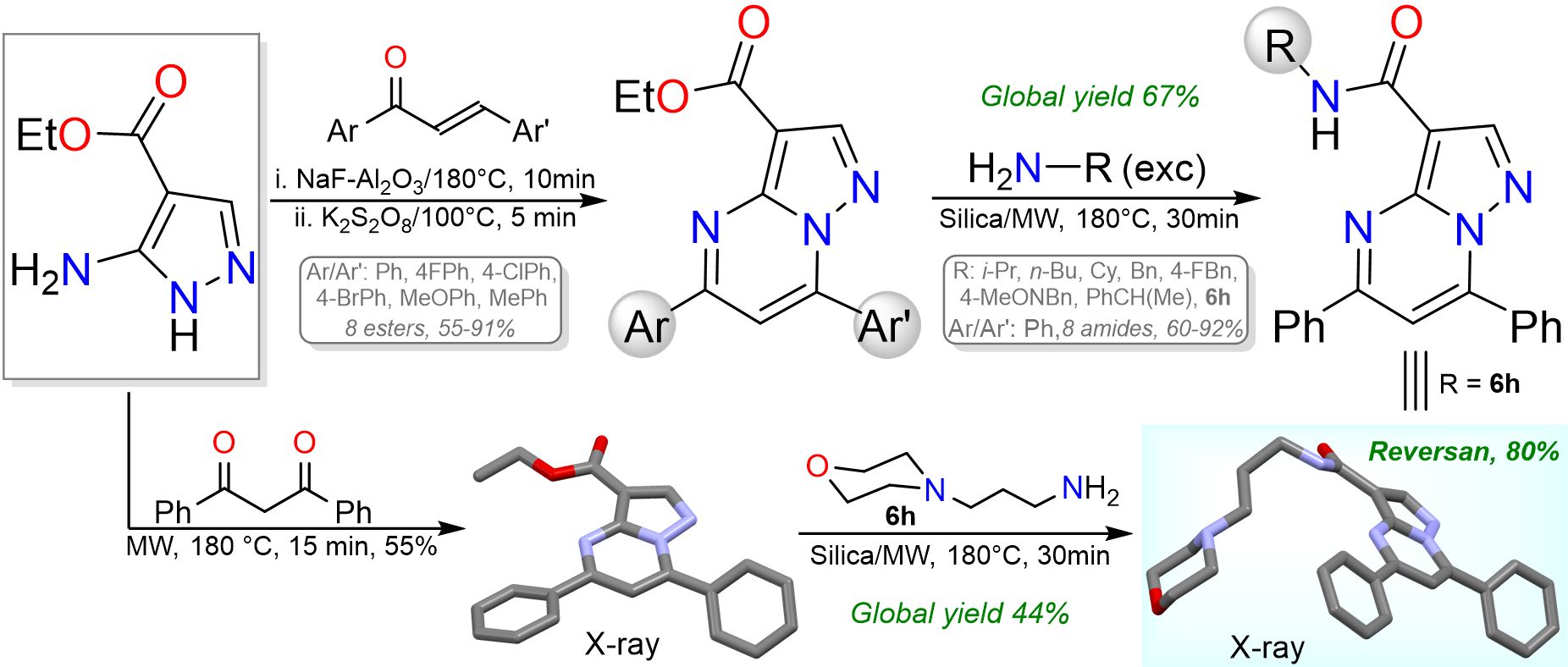
Arias-G, A.; Macías, M. A.; Portilla, J. Synthesis of structural analogues of Reversan by ester aminolysis: an access to pyrazolo[1,5-a]pyrimidines from chalcones. RSC Adv. 2023, 13, 16377-16386. https://doi.org/10.1039/D3RA02553E.



+60 1 3394999